Balancing Equations is a wonderful way to teach students about Chemistry. Balancing Equations for Chemistry can help students learn the principles of Physics and develop the basic skills needed to understand these concepts better.
In Chemistry, each chemical reaction has a property of how it will affect the surroundings around it. The property of a reaction’s reaction rate is the basis of the reaction itself. If you use a chemical reaction that is slow, you are allowing the reaction to continue at a slower rate than if you use a faster chemical reaction. This property of how quickly a chemical reaction will occur is called the reaction’s rate.
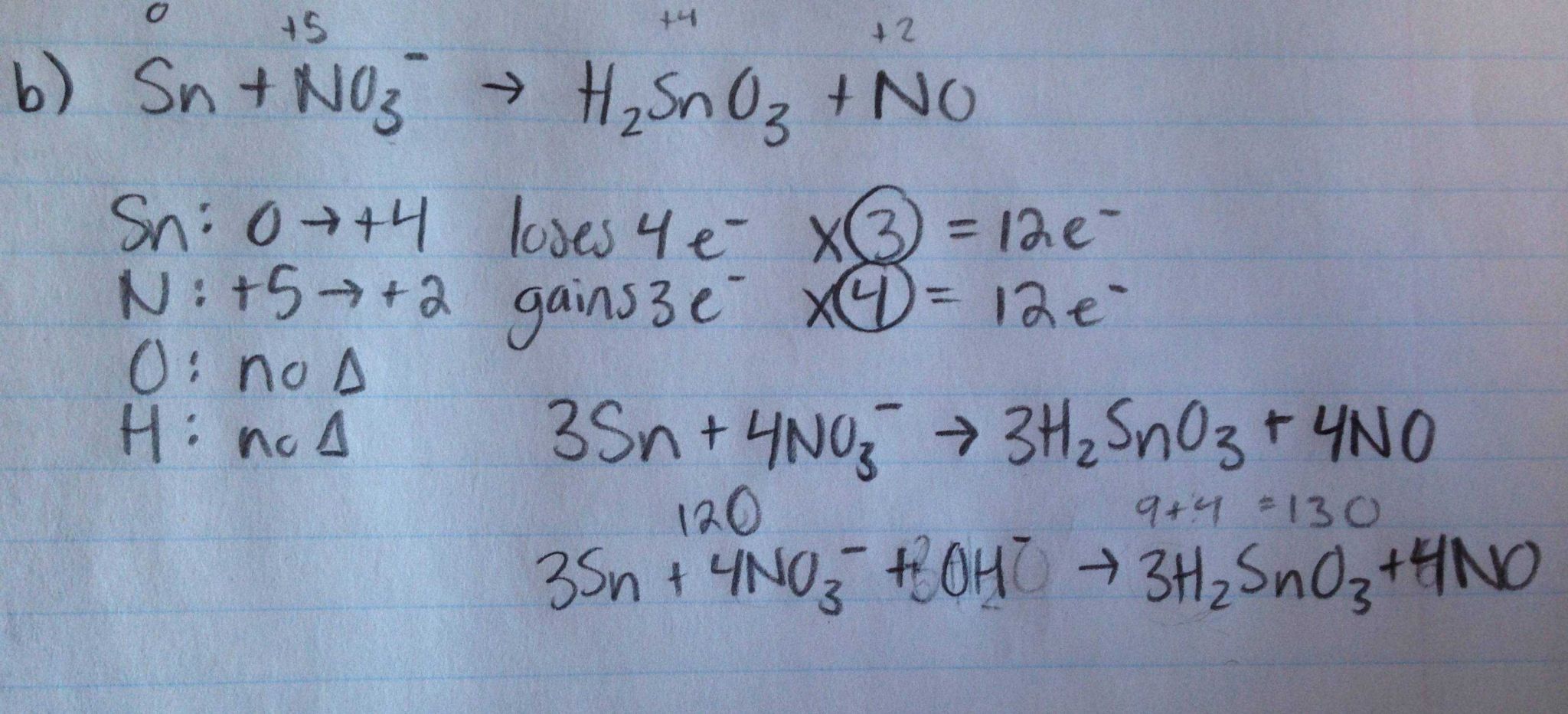
As an example, let’s take a look at the chemical reaction Fe(II) Cl(O). The first reaction is that of reacting Hydrogen into Helium, and the second reaction is of reacting Oxygen into Helium. The reason we need to make both reactions happen fast is because the reaction rate of each chemical reaction must be equal to the reactants. For example, the reaction of Hydrogen into Helium takes less time to happen than the reaction of Oxygen into Helium.
Now, if we want to know how long each chemical reaction will take, we will use the results of the first chemical reaction we wrote down. We will add the time it took to react Hydrogen into Helium and the time it took to react Oxygen into Helium together. This will give us the overall time it takes to make both reactions. If we add the two numbers together we will get the overall reaction time of the chemical reaction.
The reaction time for Fe(II) Cl(O) is almost exactly one-quarter of a second. The reaction time for Ca(I) Cl (OH) is over four hundred times greater. You can see by this example that it is necessary to take the reaction time for the reaction itself into account when working with a Balancing Equations Worksheet.
Even more important, if we want to know how long each chemical reaction will take, we must understand that the reaction itself can be stopped and restarted. For example, let’s say we wanted to add two Hydrogen atoms to an Oxygen atom. After one second, the Hydrogen atoms would have combined into one molecule, and after one second, the Oxygen atoms would have combined into another molecule.
By using a worksheet like the one we created that calculates the reaction time for a chemical reaction, we can see how the reaction itself affects the reaction time. We can also see how this affects our reaction by stopping and restarting the process. We can use this information to calculate the overall time the reaction takes to complete.
Balancing Equations for Chemistry is a great way to teach students about Chemical Reactions, and to increase their skills. Even students who have never learned about chemical reactions should still be able to use a Balancing Equations Worksheet.






