Actual yield is understood to be the quantity of the item formed by the reaction. Theoretical yield can be defined as the sum of the product formed on finishing the reaction, whenever there is no wastage of the reactants. Then if you prefer to, you can find a normal yield or yearly yield for your complete portfolio.
The quantity of solute in a particular amount of solution or solvent is called the concentration. Put simply; stoichiometry is the tradition of employing a chemical reaction equation to predict the outcomes of the reaction. Then you will correctly recognize the limiting reactant. The limiting reagent is the reagent that determines the quantity of product that may be formed using a response. Often, it’s essential to recognize the limiting reagent in an issue.
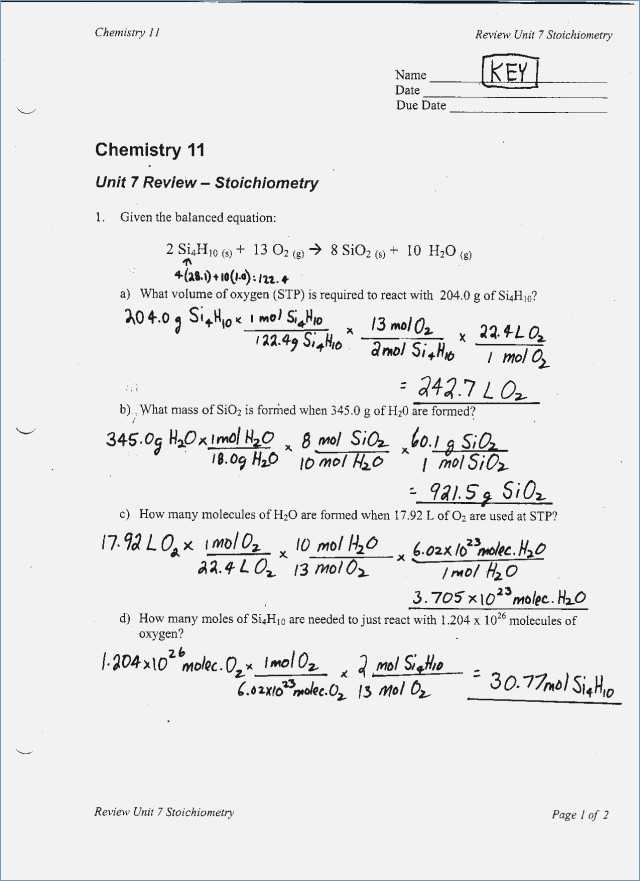
Given the balanced chemical equation which describes the reaction, there are lots of similar approaches to recognize the limiting reagent and rate the surplus quantities of different reagents. The full reaction occurs in under a second. Precipitation reactions yield a good product that’s insoluble. There’s no monster reaction to be created whenever there is just a single oxygen molecule around.
Figure out the state rule at the exact time that you figure out the federal rule to establish which one results in the decrease garnishment. In season, key decisions must be made about what things to reorder, what stuff to back off on, and the way to allocate any remaining Open-To-Buy dollars. Finding the chance for you and energy to compose your successes is perfect for the mindset. Once a sales plan was developed, the next bit of the planning procedure is to construct an inventory program. To begin with, it’s important to know the idea of STP, standard temperature and pressure.
A modest standard deviation usually means a great deal of the numbers is grouped around the center of the set. While percentage calculations are essential in nearly every area, we’ll take it from an original investment perspective henceforth in the subsequent article. Calculations which are part of a titration experiment are sure to be solution stoichiometry. Our very first step is to substitute that which we know in the equation. Chemical equations may be used to represent what the results are on either the atomic or macroscopic scale. You will learn the way to use a balanced chemical equation to compute the total amount of product formed in a chemical reaction.
The molarity formula doesn’t need to get accomplished separately as it can be included in the standard dimensional analysis setup. The ingredient that is present in less amount of moles than required is the limiting reagent for that specific reaction. All we have to know is the original and last amount of the substance under consideration.
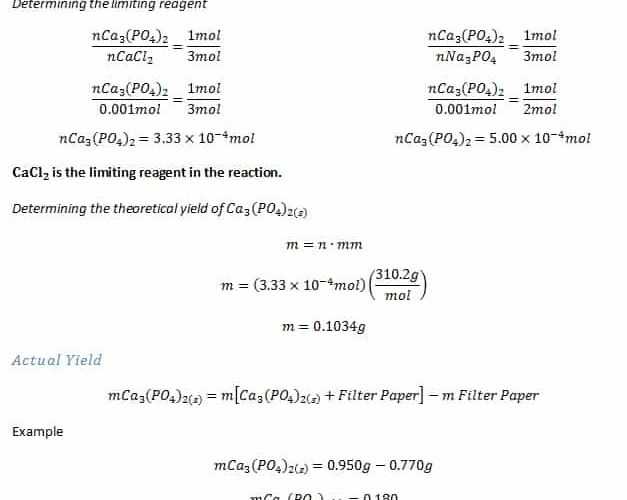
Percent recovery denotes the quantity of the original substance retained after the conclusion of the reaction. The volume and molarity given have to be employed to get the number of moles of HCl. Since you can observe, a couple of things that are possible to shift along with many others are only some of the lifetime. The procedure in which a sample dissolves in water is going to be indicated by equations like the following.