The solubility of a molecule is one of the factors affecting solubility worksheets. The solubility of a molecule in water is measured in grams per liter. The number of grams per liter depends on the kind of water, concentration and temperature. Some examples of molecules with various solubility are H 2 O, HCl, CHCl 3 and methane.
These types of molecules have high original surface area, so they will dissolve easily. Molecules with low surface area will dissolve slowly because they have low surface areas. Substances that are insoluble in water are those that have a large molecular size. Solubility worksheets also include the hydrophilic side of the molecule. Hydrophilic means that the molecule will be soluble at the water’s surface, while its hydrophobic side will make it an insoluble molecule.
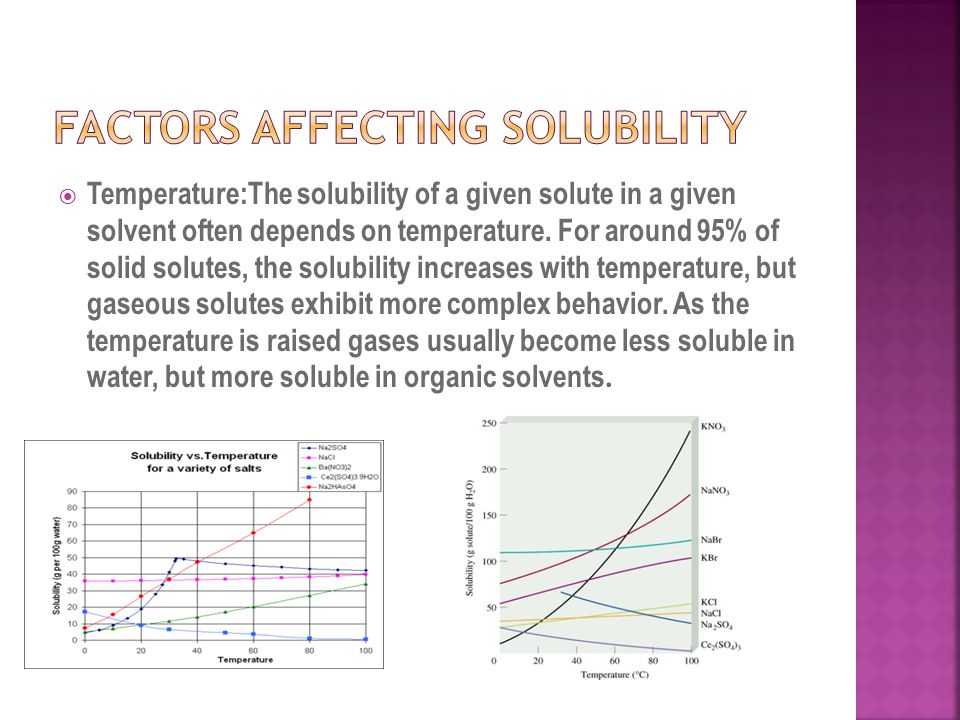
They are solubility worksheets are based on the specific gravity of the solution. Water has a specific gravity of one. Thus, this factor affects solubility worksheet answers such as – If the molecular weight of a substance is less than one, then it will be more soluble in water than the whole volume of water.
Water molecules are distributed across the surface of the liquid. In solution, molecules are dispersed and because of this, there is a constant fluctuation in the density of the water, and this has an effect on the amount of total molecules present.
The exact molecular rate of these molecules can be controlled. There are different types of water molecules such as O, Cl, NH, etc. This is in addition to a variety of chemical compounds present in the water, which have effects on the water’s solubility. These also affect the density of the water. However, all factors affecting solubility does not affect the total number of molecules present. To make a simple example, a salt can be dissolved in two parts of water, but it will not effect the total concentration.
For example, when one mixes up a complex mixture of organic compounds and water, some of the chemicals in the complex mixture would react with the water. The presence of these molecules could help the water to become more soluble. Therefore, it is necessary to separate the compounds and water solutions.
Additionally, the frequency with which one mixes up the solutions is also important. If the mix ups are done frequently, the solubility of the compound would be affected. Also, there are many different water-based products like gels, dips, body oils, soap, etc. that could be exposed to the external environment.






