Templates could be helpful after you’re trying to lose or maintain your existing weight. Despite a template, you might not have a handle on where to start. Using Worksheets indicates facilitating students to get the capability to solution questions about matters they’ve learned. The estimating worksheet is intended to direct you. This equation is known as Raoult’s Law. This equation, which is called Raoult’s law, is straightforward.
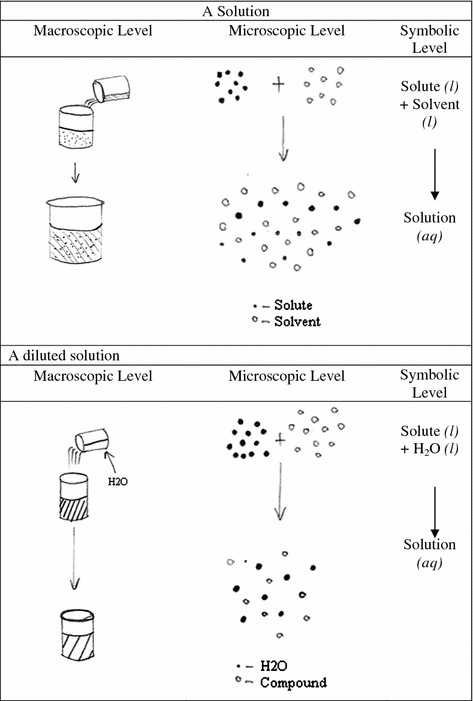
Values of and kb together with the freezing points and boiling points for a range of pure solvents are provided in the tables below. Those properties can be split into two principal groups–colligative and non-colligative properties. This property is known as boiling point elevation. Physical properties can be split into two categories. Colligative properties are determined by the number of dissolved particles, or so the solution with the increased amount of particles in solution will reveal the best deviation.
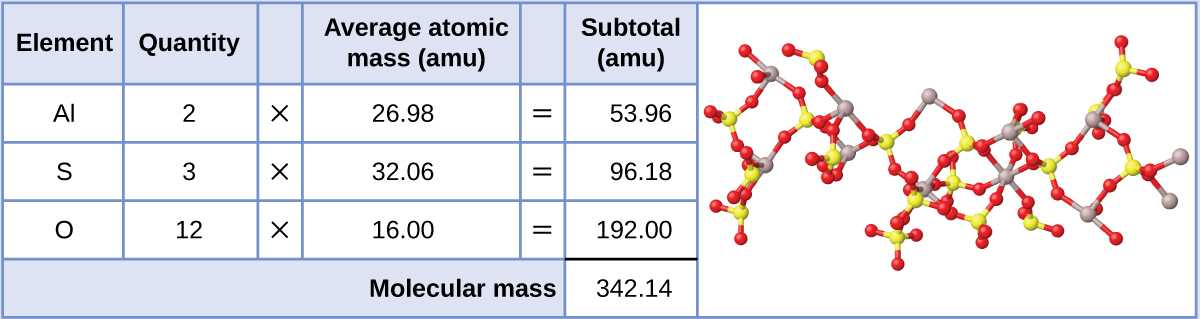
At any particular time, a person will have several things in regards to short term and long term, he wants to achieve, both. Try to remember that colligative properties are because of the variety of solute particles in the solution. There are a lot of strategies to do a cash flow program. We will need to learn the number of moles of each substance, add them together to find the entire number of moles, and after that divide to set the mole fraction of C10H8.
A standard illustration is found when salt is utilized on icy roadways. The final result is at the period of evaluation, there’s a good deal of confusion. As a consequence, liquid solutions slightly over the solvent boiling point at a certain pressure become stable, meaning the boiling point increases. Nonetheless, it decreases the speed at which the solvent molecules in the liquid can escape in the gas phase.
If both phases are at the same preliminary pressure, there’s a net transfer of solvent across the membrane into the solution referred to as osmosis. This approach is known as hemolysis. It is called crenation. That vaporization procedure is reversible. One of the absolute most important applications of colligative properties is they may be used to determine molar mass. This procedure is known as osmosis. Everything connected to the post-manufacturing procedure is included in the post category.
No matter your business planning goals, cash flow is the resource in the organization, and handling cash is the only small business purpose. Osmotic pressure explains why you ought not to drink seawater if you’re abandoned in a life raft in the center of the ocean. Besides, we know that we have to boost the vapor pressure of the liquid to be able to get to the boiling point so this must mean we will need to input more energy to reach the same vapor pressure. The pressure of the vapor phase over the liquid at this time is known as the equilibrium vapor pressure. Note that you truly have to exceed the boiling point temperature to completely evaporate the liquid. We already know this to freeze a liquid, we need to decrease the temperature. Suddenly the speed at which the water freezes decreases.