A spreadsheet can improve your accuracy. It is actually only a calculator, but with much more flexibility. Creating your initial standard spreadsheet isn’t a complicated task whatsoever. Mathematics worksheets cover the core principles in several strategies and also make sure trainees apply a wide assortment of problem addressing skills. You have the ability to learn how to fill your financial worksheet having the most acceptable numbers, and what exactly you prefer to stop. You may use the particular very same worksheet for a number of your pupils. If you don’t discover how to create an ideal language worksheet, then you’re ready to take to spelling practice worksheet template formats that are available online.
Each of these exercises describes a nuclear shift. The atomic weight of an element involves the weight of all of the isotopes. Be aware that the issue does not supply the typical atomic weight of iron.
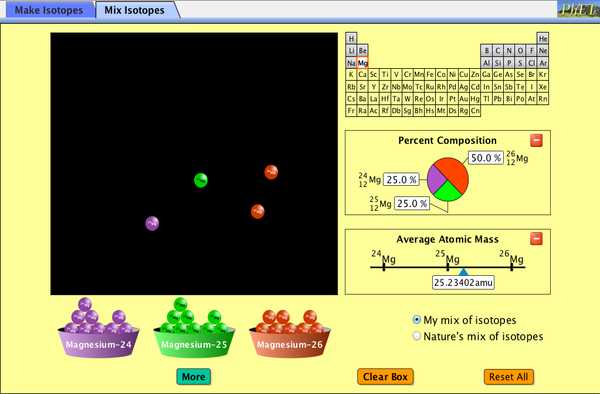
Unlike protons, the range of neutrons isn’t absolutely fixed for most elements. It is variable, resulting in isotopes, which are different forms of the same atom that vary only in the number of neutrons they possess. Frequently, the consequent number includes a decimal. In a neutral atom, the range of electrons equals the amount of protons. In an uncharged, neutral atom, the range of electrons orbiting the nucleus is equivalent to the range of protons in the nucleus. This illustration may lead you to think that atoms have exactly the same number of protons and neutrons, but an additional examination of the table above will demonstrate this isn’t true. Another illustration is lithium.
Hydrogen has three big isotopes. It has been done for you. By way of example, naturally occurring hydrogen is virtually all hydrogen-1, and naturally occurring oxygen is virtually all oxygen-16.
Atoms, as you most likely know, are incredibly tiny. In order for this to be true, atoms must be exceedingly tiny. Since they are neutral, the number of electrons in an atom is equal to the number of protons. So the electrons will be excited and we’ll speak about what excited means in only a second.
Since neutrons do not impact the charge, the variety of neutrons isn’t contingent on the range of protons and will vary even among atoms of the identical element. Isotopes are atoms which have the exact atomic number but different mass numbers as a result of change in the amount of neutrons. Comparing the proportion of the 14C concentration found in an object to the quantity of 14C in the atmosphere, the quantity of the isotope which has not yet decayed can be set. The next most typical isotope is known as deuterium. Broadly speaking, the different isotopes of each element have never been given a very different name.
It is possible to figure out the mass of each item provided that you know the chemical formulas of all of the reactants and products. In any reaction, the entire molar mass of each element involved with the reaction has to be conserved. This last particle is known as an atom. Ionizing radiation may also be particles with mass, charge, and an extremely substantial speed. Non-ionizing radiation is generated in a number of ways.