Its primary aim is to provide students with a comprehension of the basics required to understand bonding. The notion of ionic bonding developed gradually over time. There are four primary clues a chemical change has occurred. The solution can be found in the energetics of the procedure by which the compound is made. The issue with physics is you have some rules you may not break, and others you can borrow. The differences between bonds are more straightforward to spot, but they’re equally as important when you’re attempting to understand chemical bonding.
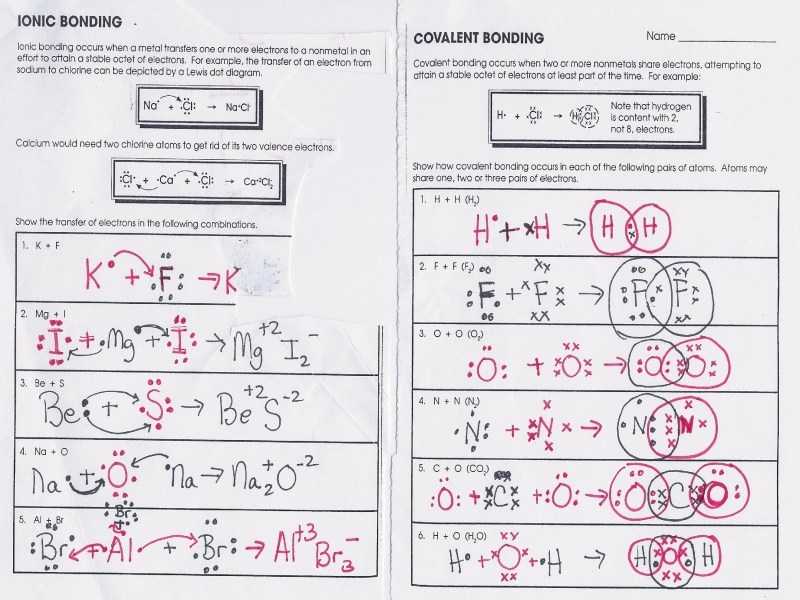
Covalent bonding requires the sharing of electrons between a couple of atoms. In such situations, the subsequent bonding often requires a description in conditions of a band structure composed of gigantic molecular orbitals spanning the whole crystal. Hydrogen bonding has a rather significant influence on the properties of water and ice. The covalent bond could be present between the similar or various atoms. Ionic bonds are essential since they let the synthesis of particular organic compounds. If you end up getting a lousy sum, then you’ve overspent and has to adapt by decreasing funds in another type or by reducing the whole amount of money created for that subsequent 30 days. You will also demand a subject to acquire total general expenses budgeted for.
Well, it takes the same quantity of energy derived from the gravity feed of the water on the other side of the hydro-wheel, since the pump would have to set the water back to the very first tank to repeat the approach. Activation energy is the extra energy that reacting substances must have as a way to participate in a chemical reaction. It requires energy to generate the high-frequency, but at the exact low power levels, it may be possible. Finally, lattice energy is the quantity of energy that is necessary to break or form ionic bonds. It cannot be determined experimentally due to the difficulty in isolating gaseous ions.
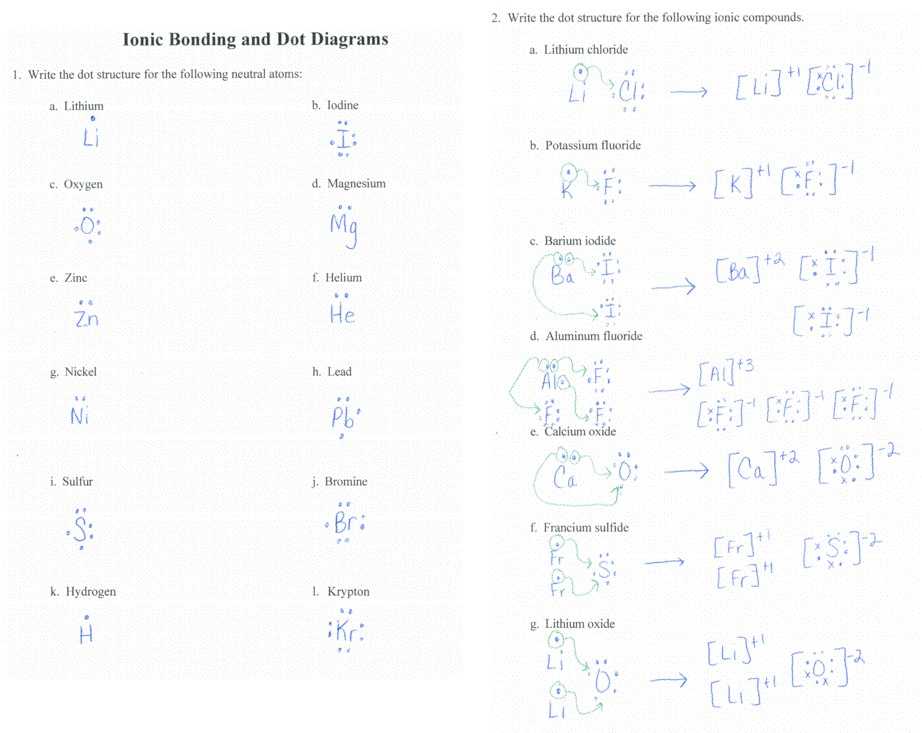
Hydrogen would have to obtain an excess electron to be like helium or lose its electron to a different atom. There are various ways in which atoms can bond together. Once you understand how many valence electrons an atom has, you may start to create molecules. By a rule of thumb, atoms in the identical group tend to behave similarly. When electrons are transferred from 1 atom to another, it is known as ionic bonding. An extra electron cannot join since it would have to come in at the fourth energy level. For a collision to work, the colliding particles have to be in the correct orientation and have to possess the vital energy to accomplish the activation energy.
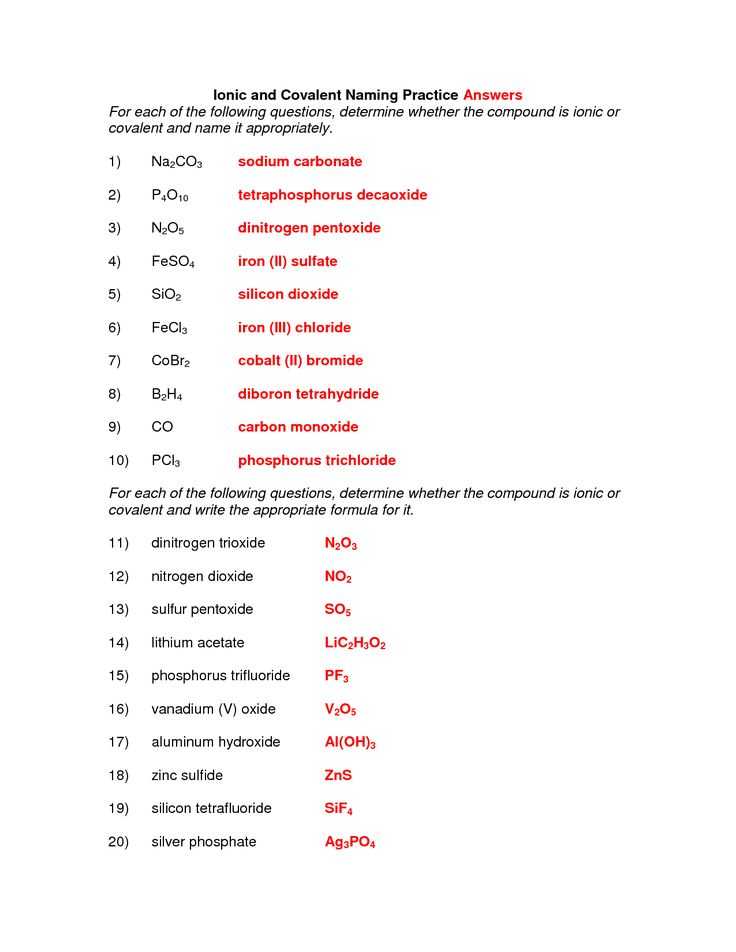
The formula identifies an exact precise compound, distinguishable from different compounds. Such compounds are called ionic compounds, instead of covalent or molecular compounds which haven’t any ionic bonds whatsoever. Molecular compounds incorporate the range of atoms in the name using a prefix. Most ionic compounds tend to dissociate in polar solvents because they’re often polar. A Lewis-Dot Structure may be used to reveal the bonding between atoms and the number of electrons are in their valence layer. When you examine the structure and characteristics of each, some factors permit them to act in a particular way, in the same way, the sodium element, and chlorine element interacts together. The cross-over way is demonstrated.